How to Properly Calculate pH: Essential Methods for Accurate Results in 2025
Understanding and effectively calculating pH is crucial in various scientific fields, including chemistry, biology, and environmental science. The pH scale provides a measure of acidity and alkalinity, which in turn plays an essential role in understanding chemical reactions and biological processes. In this article, we will delve into how to calculate pH accurately, explore various pH calculation methods, and highlight the importance of pH measurement for both laboratory practices and real-world applications.
Understanding the pH Scale and Its Importance
The **pH scale** is a logarithmic scale ranging typically from 0 to 14, which quantifies the level of acidity or basicity in a solution. A pH of 7 indicates neutrality, values below 7 indicate acidic solutions, and values above 7 indicate basic (alkaline) solutions. Understanding this scale is vital, as it affects various properties of solutions, including solubility, color, and chemical reactivity. Knowledge of **importance of pH** extends further into fields like agriculture, where soil pH can dramatically influence plant health. For instance, certain plants thrive in acidic environments while others require more alkaline conditions.
Key Components of the pH Scale
While discussing the **pH and acidity** concept, it is essential to recognize the nuances within this scale. **Acidic solutions** have a higher concentration of hydrogen ions (H+), which is directly related to a lower pH value. Conversely, **basic solutions** have fewer hydrogen ions and can result in a higher pH value. Understanding these relationships allows scientists to manipulate conditions to achieve desired outcomes, such as promoting plant growth or enhancing chemical reactions. Moreover, a precise understanding of the **pH value range** enhances our ability to predict chemical behavior in reactions and inform regulatory measures concerning environmental quality.
The Role of pH in Biological Systems
The significance of pH also extends into **pH in biological systems** where enzymes function optimally within specific pH ranges. For instance, human stomach enzymes work best in highly acidic conditions (around pH 2), whereas other enzymes in the bloodstream require more neutral conditions. Understanding how to measure pH accurately can not only improve biochemical experiments but also assist in medical diagnostics and treatments by understanding the body’s acid-base balance.
Methods of pH Measurement
Several **pH testing methods** are available today, ranging from simple litmus paper tests to advanced electronic systems. Each method has its advantages and specific application scenarios. Choosing the right technique involves understanding the **factors affecting pH** stability, the precision required, as well as the specific context in which pH measurement is being carried out, such as environmental testing or food science.
Using a pH Meter
A **pH meter** is one of the most accurate and widely used instruments for measuring pH levels. These meters function based on a glass electrode that responds to H+ ion concentration. When using a pH meter, it is crucial to **calibrate a pH meter** accurately to ensure reliable results. Calibration involves using buffer solutions of known pH values, typically around pH 4.01, 7.00, and 10.01, to adjust the device. Failing to calibrate correctly may lead to **sources of error in pH measurements**, which can cascade into faulty conclusions in research or practical applications.
Manual Calculation of pH
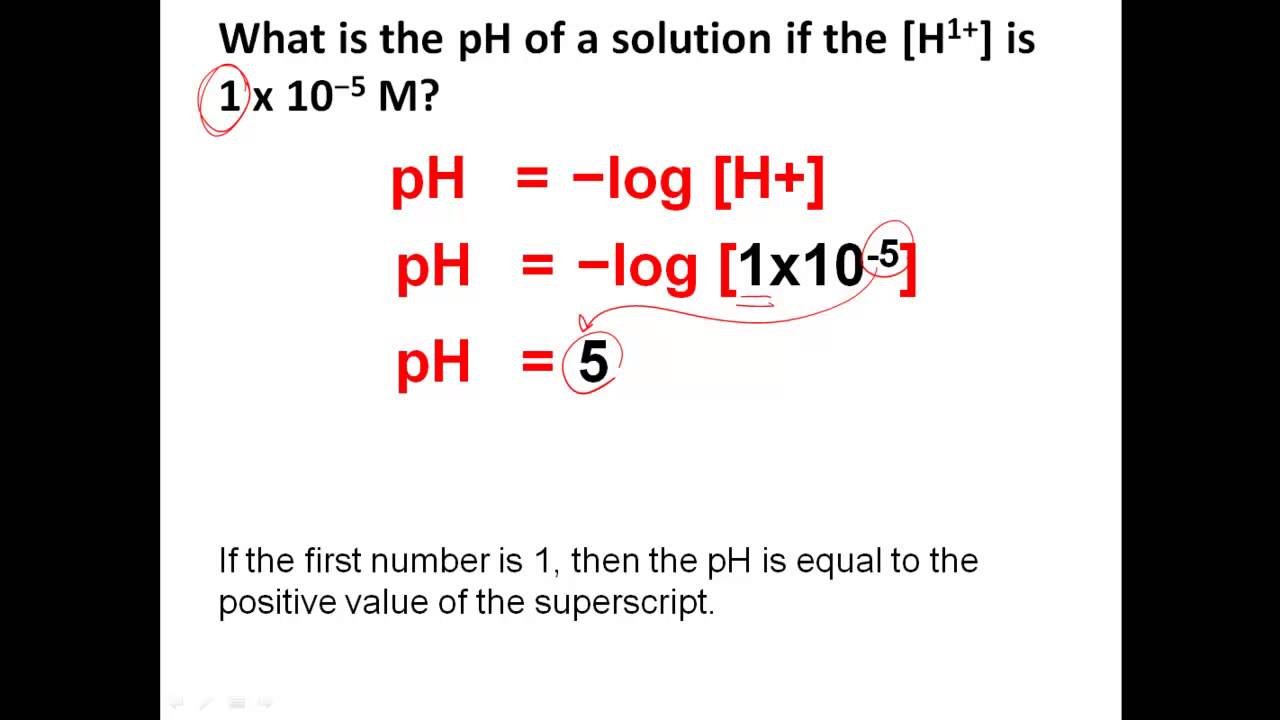
For educational purposes or in situations where sophisticated electronic equipment isn’t required, manual calculation remains a valuable approach. The formula for **calculating pH of solutions** is given by pH = -log[H+]. To derive this value accurately, the concentration of hydrogen ions can be determined chemically or via titration. Titration not only helps in obtaining pH levels but also provides a pH titration curve that graphically represents the relationship between pH and analyte concentration. This process enhances understanding of the **acid-base neutralization** and allows for determination of endpoints during titrations.
Applications and Considerations of pH in Various Fields
The impact of pH measurement stretches across many domains, including chemistry, agriculture, and even health. Different industries are coming to terms with the implications of pH and its interplay with various factors.
pH in Agriculture
In agriculture, **pH in soil** is crucial, as it directly impacts the availability of nutrients to plants. For example, if the soil pH is too low or too high, crops may not absorb essential minerals effectively, leading to reduced yields. Farmers often conduct regular soil pH tests to monitor and adjust soil conditions, ensuring optimal conditions conducive to growth. Techniques such as **adjusting pH levels** using lime for acidity or sulfur for alkalinity are prevalent practices among agriculturalists.
pH and Water Quality
**Water quality pH** is another critical consideration in pollution management and aquatic ecosystems’ health. Aquatic organisms require specific pH levels to thrive; therefore, testing the **pH in swimming pools**, rivers, and lakes is essential for both environmental monitoring and recreational safety. Variations in pH levels can indicate anthropogenic impacts affecting ecosystems, prompting necessary interventions to restore balance. Understanding the **health impact of pH** levels in drinking water is equally vital; excessively acidic or basic water can lead to corrosion or mineral leaching.
Best Practices for Accurate pH Measurement
To achieve robust and reliable pH measurements, several best practices should be implemented in various testing scenarios. Understanding the **technical aspects of pH testing** is key to minimizing discrepancies and ensuring excellent accuracy.
Regular Calibration and Maintenance
Consistent **calibrating** of pH meters and maintaining them in good condition is fundamental in any laboratory setting. Environmental conditions, electrode longevity, and usage frequency can impact measurements. Professionals often recommend frequent check-ups to suit specific laboratory environments and material being measured. Keeping a list of **standard solutions for pH measurement** for reference can streamline this process significantly.
Usage of pH Indicators
Panchromatic or universal **pH indicators** provide a visual representation of pH levels and can be particularly useful in educational settings. These indicators change color based on the pH level of a solution, allowing students to directly connect theory with observable phenomena—an effective educational tool. However, visual indicators are not as precise as electronic meters and are usually more appropriate for approximate pH assessments.
Key Takeaways
- The pH scale is crucial for understanding acidity and alkalinity in various solutions.
- Different methods are available for accurately measuring pH, such as pH meters and manual calculation techniques.
- pH heavily influences agriculture, water safety, and various chemical and biological processes.
- Regular calibration and maintenance of pH measurement devices ensure reliability.
- Visual indicators can be useful educational tools, but electronic methods provide higher accuracy.
FAQ
1. What are the common applications of pH measurement?
pH measurement is used in diverse fields, including agriculture for soil management, in pharmaceuticals to ensure medication stability, and in environmental science to monitor water quality and pollutant levels. Without understanding pH roles, it would be challenging to maintain plant health, carry out chemical reactions, or ensure public health.
2. How does pH affect enzyme activity?
The activity of enzymes is highly dependent on environmental conditions, particularly pH. Each enzyme operates optimally at a specific pH range; deviations can lead to altered activity levels, potentially affecting metabolic rates and overall physiological functions.
3. What role does pH play in fermentation?
During fermentation, **pH levels** greatly influence the production of acids and alcohol by microorganisms. An optimal pH fosters a conducive environment for yeast and bacteria, ensuring that fermentation processes are efficient and yield desirable products.
4. How can I adjust pH levels in soil?
Adjusting soil pH involves adding amendments such as lime to raise pH or sulfur to lower it. Understanding the specific pH needs of crops and conducting regular soil tests enable farmers to make informed decisions about their soil management practices.
5. Are there portable devices for measuring pH?
Yes, portable pH measuring devices such as hand-held pH meters and test strips are widely available. These solutions are particularly useful for field sampling, water testing, and ensuring accurate measurements in various settings outside of a laboratory environment.
6. What are common errors when measuring pH?
Common errors include not calibrating the pH meter regularly, using contaminated solutions, not immersing the electrode properly, and failing to maintain equipment properly. These inaccuracies can lead to incorrect pH readings, affecting experimental results.
7. How does the relationship between pH and concentration work?
The relationship between pH and concentration of hydrogen ions is logarithmic, where each whole number change on the pH scale represents a tenfold change in H+ ion concentration. Understanding this relationship is essential for predicting behaviors in chemical reactions and optimizing various processes.