How to Effectively Find Percent Yield in 2025: A Smart Guide to Enhance Your Chemistry Skills
Understanding and calculating percent yield is a fundamental topic in chemistry that every student and professional should master. The percent yield formula is used to measure the efficiency of a chemical reaction by comparing the actual yield obtained from an experiment to the theoretical yield predicted by stoichiometry. In this article, we will explore effective methods to calculate yield and practical examples to solidify your understanding.
Understanding Percent Yield and Its Significance
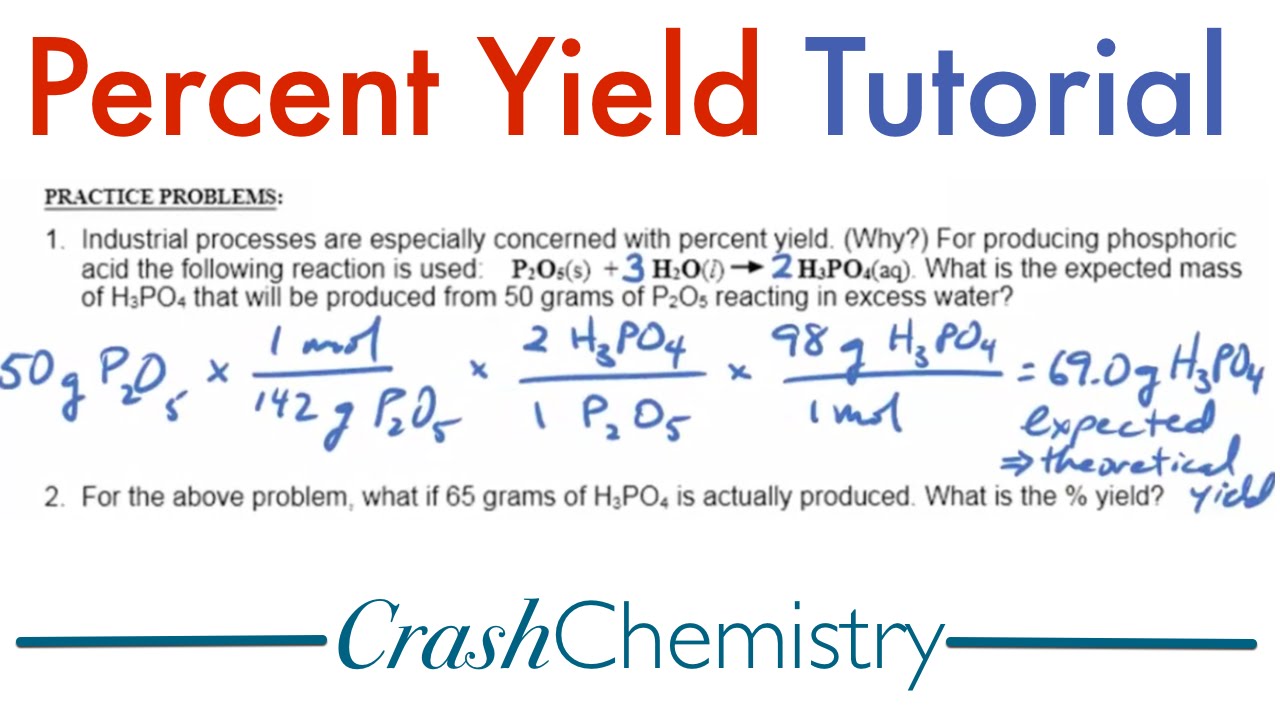
Percent yield is a crucial concept in the field of chemistry. It not only indicates the success of a chemical reaction but also helps students and professionals gauge their laboratory results. The formula for calculating percent yield is:
Percent Yield = (Actual Yield / Theoretical Yield) × 100%. In this formula, the actual yield represents the quantity of product obtained from an experiment, while the theoretical yield is the maximum amount of product that could be generated based on the reactants used.
Components of Yield Calculation
When calculating yield, it’s essential to understand its primary components. The actual yield is usually determined from empirical data collected during the experiment. To ensure accuracy, careful measurements should be taken using analytical balance to avoid errors in the yield determination. On the other hand, the theoretical yield is calculated using stoichiometric ratios from the chemical equation involving the limiting reactant.
Common Problems in Yield Calculation
In chemistry, yield problems often arise due to various factors such as incomplete reactions or side reactions that divert reactants. These errors affect the overall chemistry yield. Identifying these limiting factors can significantly improve the yield efficiency. A methodical approach, such as performing a mass balance, allows chemists to analyze discrepancies in yield efficiently.
Practical Examples of Percent Yield Calculation
Let’s go through an example to illustrate how to calculate percent yield. Suppose you conducted a reaction where the theoretical yield of a compound was calculated to be 50 grams; however, you only obtained 40 grams. Applying the percent yield formula: Percent Yield = (40/50) × 100% = 80%. This result showcases that while your reaction was successful, there is still room for optimization, reflecting our need to embrace continuous improvement in laboratory techniques.
Factors Affecting Percent Yield in Reactions
The calculation of chemical reaction yield can be influenced by several factors. Understanding these influences can help optimize the yield in chemistry experiments.
Temperature and Pressure Influences
Temperature and pressure play significant roles in chemical reactions. Increased temperature often accelerates reaction rates, favorite for reactions with reactants that possess higher activation energy. On the flip side, high pressure is essential for reactions involving gases, which can optimize product formation. Careful adjustment of these variables can lead to better yield outcomes.
Purity of Reactants
The purity of starting materials is critical. Impurities can lead to deviation in yield and hinder the reaction efficiency. Ensuring high-grade reactants can contribute significantly to maximizing your desired yield. Conducting a thorough quality assurance check on materials before beginning your experiments is highly recommended.
Stirring and Mixing Efficiency
Proper mixing can drastically impact reaction yield. Insufficient mixing can lead to uneven distribution of reactants, thereby limiting product formation. \[Yield troubleshooting\] often involves ensuring that all reactants are adequately meshed, which enhances reactant conversion while simultaneously ensuring compliance with the material balance.
Calculating Percent Yield: A Step-by-Step Guide
To deeply comprehend how to calculate yield, practical application is key. Here’s a streamlined guide to assist in calculating yield in chemistry.
Step 1: Gather Your Data
Begin by collecting all necessary data. This includes measurements of both the actual yield obtained from your experiments and the theoretical yield determined through calculations of stoichiometry. Make sure to note any deviations or errors that might have occurred during the reaction.
Step 2: Use the Formula
Employ the formula Percent Yield = (Actual Yield / Theoretical Yield) × 100% to compute your yield. Substituting your collected values will give you a clear indication of the success rate of your experiment.
Step 3: Analyze and Document Results
Once you calculate the percent yield, document your findings within your laboratory reports. This analysis should include reflection on factors that could have influenced the yield, observational data from experiments, and proposals for improving yield outcomes in future experiments.
Key Takeaways
- The calculation of percent yield is vital to assess the efficiency of a chemical process.
- Understanding factors affecting yield determination can help enhance outcomes in experiments.
- Common practical applications and problems encountered during yield calculations can aid in refinement and optimization.
FAQ
1. What is the definition of percent yield?
Percent yield is a measurement that expresses the efficiency of a chemical reaction. It is defined as the ratio of the actual yield, what is produced in an experiment, to the theoretical yield, the maximum possible amount that could be produced based on stoichiometric calculations, all multiplied by 100%.
2. How can I improve my yield in chemistry experiments?
To enhance yield, focus on optimizing conditions such as temperature, pressure, and purity of reactants. Additionally, ensure proper mixing and consider performing reactions under controlled environments. Adjusting these variables can lead to more efficient reactions and improve overall yield performance.
3. What factors can cause deviations in percent yield?
Deviations in percent yield can often occur due to inconsistencies in the purity of reactants, incomplete reactions, side reactions consuming reactants, or experimental errors such as incorrect measurements. Identifying and refining these issues can lead to more accurate yield determinations.
4. Can theoretical yield exceed 100%?
No, the theoretical yield cannot exceed 100%. If it appears so, this may result from errors in calculation or measurement, or the formation of more than one product from the reaction. It’s important to validate your calculations and ensure accurate measurements.
5. Why is yield analysis important in chemical reactions?
Yield analysis is crucial as it helps determine the effectiveness and efficiency of a chemical reaction. This can provide insight into potential improvements for future experiments, assist in research and development processes, and play a critical role in industries where product efficiency is essential.