How to Properly Name Ionic Compounds in 2025: A Simple Guide to Understanding Ionic Compounds Naming
The Fundamentals of Naming Ionic Compounds
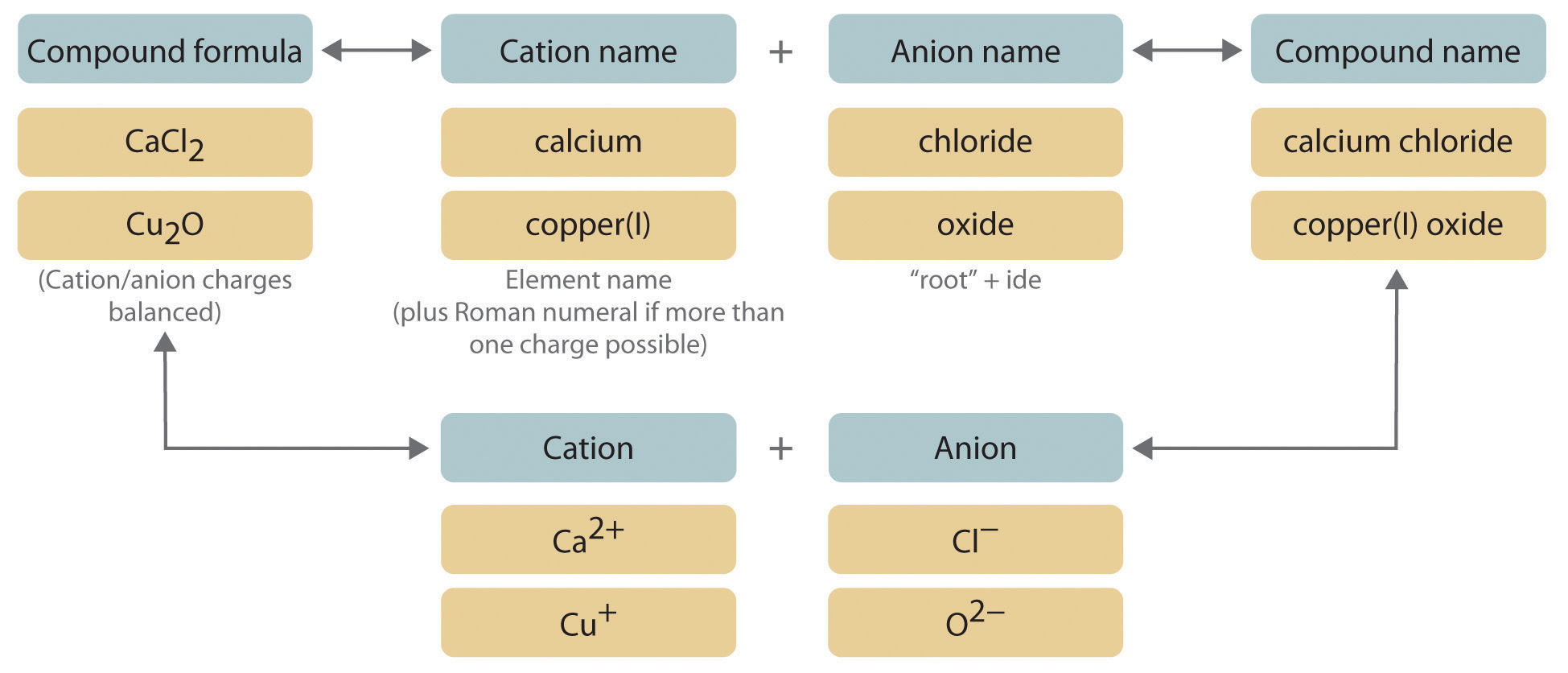
Naming ionic compounds involves a series of rules and systematic approaches that remain crucial for clarity in the field of chemistry. This process, known as **ionic compounds naming**, helps in identifying compounds formed by the reaction between metals and non-metals. Understanding these rules allows chemists and students alike to communicate compound identities accurately. The foundation typically starts with recognizing the components of ionic compounds: cations (positively charged ions) and anions (negatively charged ions), whose names contribute directly to the formulation of the compound’s name.
Identifying Cations and Anions
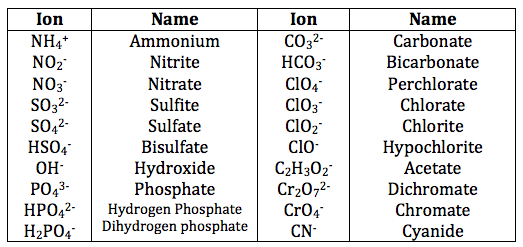
The first step in properly **naming ionic compounds** involves knowing the **cation and anion examples**. Cations, generally metals like sodium (Na⁺), calcium (Ca²⁺), and transition metals such as iron (Fe²⁺ or Fe³⁺), vary in their charges, particularly with transition metals. They are often followed by anions, which can be monatomic (like chloride Cl⁻) or polyatomic (like nitrate NO₃⁻). For instance, in sodium chloride (NaCl), sodium acts as the cation, while chloride is the anion. A grasp of the **rules for naming ionic compounds** also includes familiarity with the **name of ionic compounds chart**, helping quickly identify common ions and their respective groups.
Understanding Ionic Bonding
One must also comprehend the essence of **identifying ionic compounds**. Ionic bonds form when electrons are transferred from a metal to a non-metal, creating a stable charge distribution. This contrasts with covalent bonding, where electrons are shared. Therefore, knowing the basic properties of whatever compound you are handling can influence the naming conventions applied. For instance, properly identifying a sulfate (SO₄²⁻) when it combines with calcium forms calcium sulfate (CaSO₄), instead of overlooking the polyatomic ion’s specific characteristics.
Systematic Naming Conventions
The **systematic naming of ionic compounds** adheres to established conventions, primarily governed by **naming conventions in chemistry** based on the IUPAC guidelines. These help to maintain consistency and clarity in the field. The steps involved typically entail determining the cation followed by the anion, ensuring the correct oxidation states are expressed.
Anion Suffix Changes
An important aspect of naming involves understanding **anion suffix changes**. For instance, when naming a compound like K₂SO₄, the sulfate ion (SO₄²⁻) dictates the anion’s name, which remains consistent. In contrast, if oxygen is removed from a polyatomic ion, the suffix changes from “-ate” to “-ite”. Thus, sulfite is SO₃²⁻ while the sulfate remains SO₄²⁻. Grasping these changes is fundamental to constructing the right nomenclature. Additionally, this naming rule applies uniformly across similar ions, such as in chlorates and chlorites.
Naming Compounds with Variable Valency
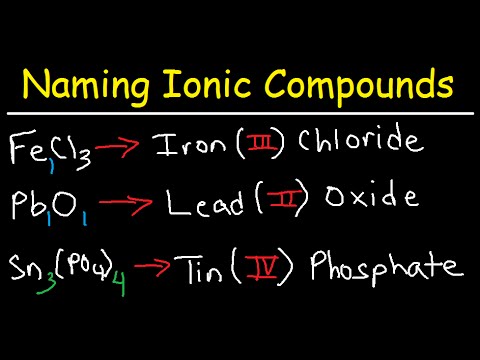
When dealing with transition metals that exhibit different valencies, it’s essential to include this valency in the name of the compound. For **naming compounds with variable valency**, chemists often use Roman numerals to denote the charge of the cation, such as lead (II) oxide (PbO). This practice enhances clarity and helps avoid misunderstandings about the specific compound’s structure. Using examples of ionic compounds that highlight these rules can assist in elucidating how diversification in naming occurs across varying components.
Common Mistakes in Ionic Naming
<pWhen learning **naming ionic compounds**, it's common to encounter pitfalls. Awareness of these **common mistakes in ionic naming** can dramatically enhance one’s comprehension and ability to articulate chemical names effectively.
Simple Ionic Compound Names
A frequent error is the incorrect assumption that naming **simple ionic compounds** follows all the same rules without accommodating the needs of polyatomic ions, like ammonium (NH₄⁺). For example, incorrectly labeling ammonium sulfate as merely a combination of ammonium and sulfate without familiarity with the nuances of these ions can lead to miscommunication. Thus, knowing when to integrate or separate these charged particles into their systematic names is pivotal.
Best Practices for Naming Ionic Compounds
The **best practices for naming ionic compounds** also emphasize consistency in format and clarity. One should always start with the cation’s name followed by that of the anion. For example, in the case of **potassium hydroxide (KOH)**, the potassium (K⁺) cation is presented first, collectively understanding the nature of the hydroxide (OH⁻) anion. Such practices simplify the identification and writing of ionic formulas for compounds.
Naming Ionic Compounds with More Than Two Elements
To navigate more complex structures, we often need to address how to name ionic compounds comprising more than two elements, commonly seen in the realm of chemical nomenclature. Utilizing polyatomic ions expertly is essential here, thus **naming compounds with more than two elements** hinges on grasping the core of what each component of the ionic structure contributes to the compound.
Ionic Naming Conventions With Hydrogen
In instances where hydrogen is involved in the compound, such as in **naming ionic compounds with hydrogen**, the process becomes slightly more intricate. For example, in sodium bicarbonate (NaHCO₃), we refer to sodium and bicarbonate, recognizing that hydrogen isn’t just a filler but actively contributes to the compound’s acid-base properties. Implementing the rules for naming ionic compounds focusing on hydrogen aids in understanding their role in both biological systems and ionic solutions.
Practice Makes Perfect: Naming Practice for Ionic Compounds
Finally, engaging with **naming practice for ionic compounds** through various quizzes and exercises allows learners and chemists to refine their knowledge continuously. Interactive activities assist in solidifying the fundamentality of ions, their charges, and the intricacies of naming conventions relevant to both simple and complex ionic structures. Effective practice encourages retention of these significant naming structures, fostering a more profound comprehension of chemistry overall.
Key Takeaways
- Understanding the basics of **ionic compounds naming** involves identifying cations and anions, along with their conventions.
- Systematic naming follows established rules, with specific attention to **anion suffix changes** and variable valency, especially in transition metals.
- Avoiding common mistakes, especially with simple and complex ionic substances, is crucial in precise chemical communication.
- Engaging in **naming compounds with more than two elements** and having practical naming exercises can distinctly enhance one’s understanding.
FAQ
1. What are ionic compounds?
Ionic compounds are formed through the electrostatic attraction between cations and anions. These compounds possess high melting and boiling points, are typically soluble in water, and can conduct electricity when dissolved or melted, demonstrating their characteristic ionic dominating properties.
2. Are there exceptions in ionic naming rules?
Yes, exceptions do exist; for instance, some transition metals can exhibit more than one oxidation state, leading to unique naming conventions. Moreover, certain polyatomic ions may have unusual naming behaviors, like sulfate compared to sulfite. It’s vital to consult updated references to ensure proper identification.
3. How do I practice naming ionic compounds effectively?
Effective practice comes from engaging with quizzes that challenge you to name various ionic compounds, such as those found in chemistry workbooks or online educational resources. Using a **checklist for naming ionic compounds** can also be beneficial as it ensures adherence to all necessary rules.
4. What are common polyatomic ions to remember?
Common polyatomic ions include acetate (C₂H₃O₂⁻), sulfate (SO₄²⁻), and nitrate (NO₃⁻). Particularly, memorizing these ions assists consistently in **naming ionic compounds**, especially those including multiple components.
5. How does one identify cation and anion charges?
Cation and anion charges can be identified utilizing the periodic table, where group numbers often correspond to typical charges, and recognizing compounds that follow specific oxidation states listed in reference materials, ensuring a solid foundation when naming ionic compounds correctly.
For more comprehensive learning and to engage further on this topic, visit this resource and explore additional insights.